OUR SCIENCE
For more than thirty years, numerous immunotherapy strategies have been investigated aimed at enhancing the immune system to achieve specific antitumor effects while avoiding immune escape mechanisms. In recent years, the most promising immunotherapy has been the development of the live drug called CAR-T cells, providing a new option to relapsed/refractory hematological patientss apart of surgery, chemotherapy and radiation therapy
What are CAR-T cells?
T cells are a type of white blood cell from the immune system that exert a natural antitumor potential and are able to detect and eliminate cancerous cells, how ever, this tumoral cells can escape from T cells. Chimeric Antigen Receptor (CAR)-T cell therapy has been revolutionary as it has produced remarkably effective and durable clinical responses. CARs are engineered synthetic receptors that function to redirect lymphocytes, most commonly T cells, to recognize and eliminate cells expressing a specific target antigen. CARs consist of four components: (1) an extracellular target antigen-binding domain, (2) a hinge region, (3) a transmembrane domain, and (4) one or more intracellular signalling domains, and, depending on the number and type of intracellular domains, the CAR-T are divided into generations.
A tool is needed for the delivery of the foreign gene into human cells. At present, the major vectors for gene therapy in basic research and clinical study are viruses. The viral vectors include retroviruses (including lentivirus), adenovirus and adeno-associated virus. Among them, the most popular tools for gene delivery are genetically engineered retroviruses
These modified T cells to recognize tumors more efficiently have shown great success in hematological malignancies, but there are still problems to be solved. Since they have only been successfully for blood tumors, around 50% of these patients do not respond satisfactorily and relapse after 1-year post CAR-T therapy, they have not yet been applied successfully to solid tumors, and severe side effects also appear. The major side effect in patients treated with CAR-T cells is Cytokine Release Syndrome (CRS). Althought this side effects can be managed with currently available interventions, it’s important to reduce this side effects while maintaining the effycacy of the treatment.
Our aproach
We believe that our approach can overcome the current hurdles in CAR-T cell therapy in the treatment of refractory hematological tumors and in solid tumors, where there is a large gap while reducing the number and severity of side effects through the use of our two technologies TCR-like and Lent-On-Plus .
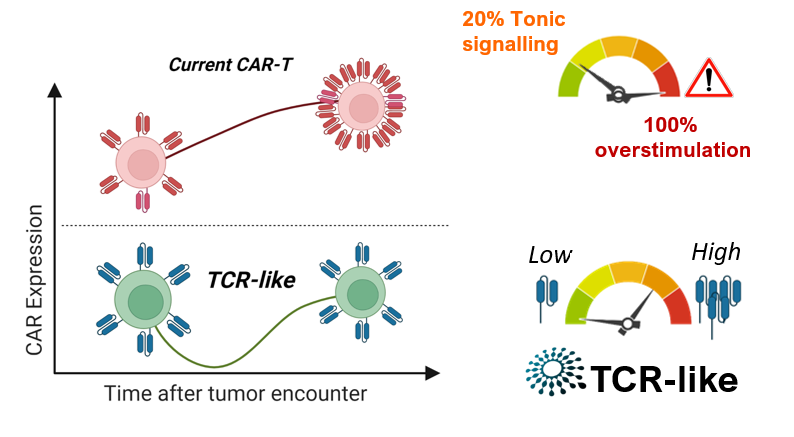
TCR-like is the first platform that allows the physiological expression of the CAR receptor using viral vectors. We propose something new and unique to minimize the lack of efficacy and side effects. TCR-like represents a creative approach to the expression of any gene with activity in immunotherapy, including CAR, mimicking the expression pattern of the TCR (T cell receptor). Unlike the current CAR-T cell therapy, which increase the expression of the CAR receptor in the membrane, producing an overstimulation that triggers premature exhaustion and failire of CAR-T cell’s fitness
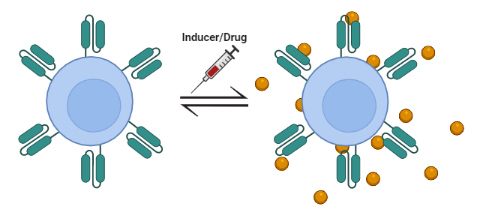
Lent-On-Plus is, due to its characteristics, possibly the only system on the market that allows doxyxyxline-inducible expression with clinical application possibilities. Of particular relevance is its ability to generate 4th generation inducible CAR-T cells (iTRUCKs). These products would make it possible to approach the treatment of aggressive lymphomas and solid tumors, where CAR-T therapy is currently not effective. The fact of controlling the expression of highly active immunomodulatory molecules reduces the potentially devastating effects caused by a constitutive expression of these molecules.
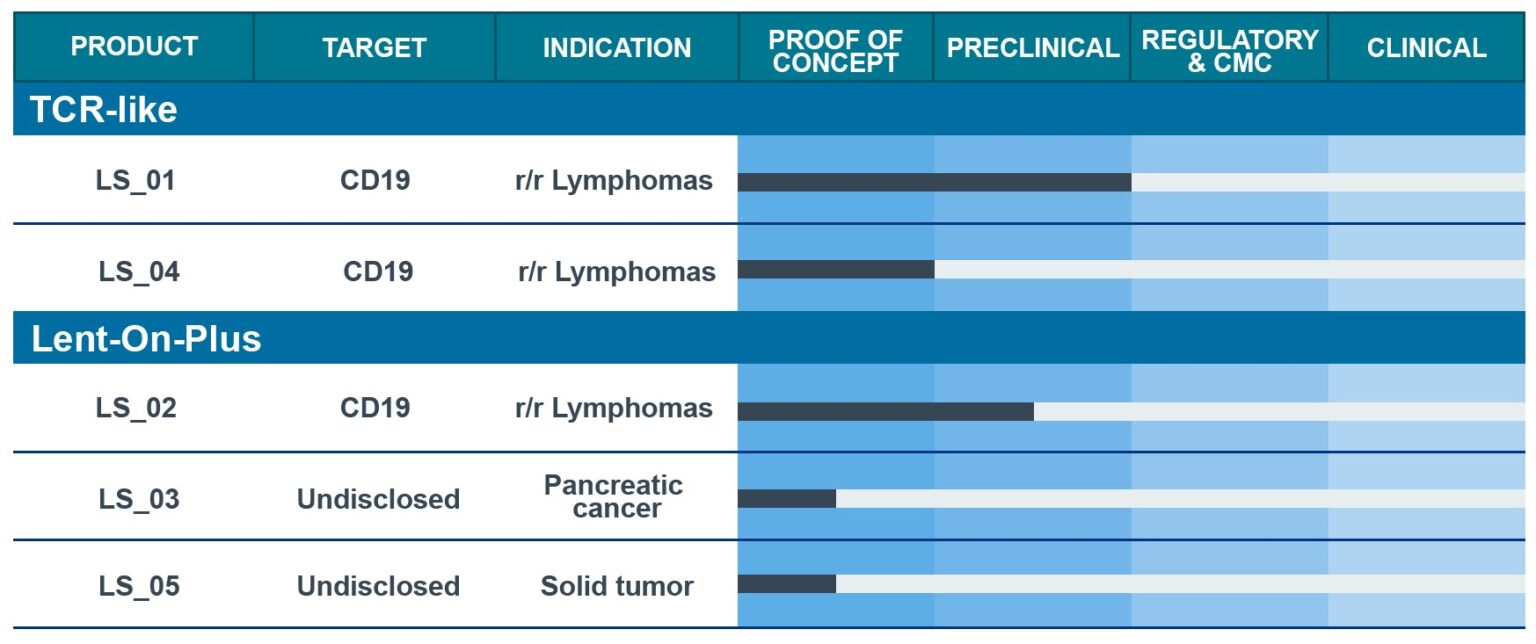
OUR PIPELINE
LentiStem Biotech is building a diversified pipeline of enhanced and safer CAR-T candidates using pre-clinical and clinical assays to demostrate its ability to improve current CAR-T cell therapies
publications
Lentiviral vectors for inducible, transactivator-free advanced therapy medicinal products: Application to CAR-T cells
Molecular therapy. Nucleic acids vol. 32 322-339. 28 Mar. 2023, doi: 10.1016/j.omtn.2023.03.018
Physiological lentiviral vectors for the generation of improved CAR-T cells
Molecular therapy oncolytics vol. 25 335-349. 18 May. 2022, doi: 10.1016/j.omto.2022.05.003
Externally-Controlled System for Immunotherapy: From Bench to Bedside
Front Immunol. 2020 Sep 4;11:2044. doi: 10.3389/fimmu.2020.02044. eCollection 2020
The IS2 Element improves transcription efficiency of integration-deficient lentiviral vector episomes
2018. Molecular therapy. Nucleic acids, 13, 16-28. doi: 10.1016/j.omtn.2018.08.007
Lent-On-Plus Lentiviral vectors for conditional expression in human stem cells.
2016. Scientific reports, 6, 37289. doi: 10.1038/srep37289
A chimeric HS4-SAR insulator (IS2) that prevents silencing and enhances expression of lentiviral vectors in pluripotent stem cells
PLoS One. 2014 Jan 6;9(1):e84268. doi: 10.1371/journal.pone.0084268
Development of an all-in-one lentiviral vector system based on the original TetR for the easy generation of Tet-ON cell lines
PLoS One. 2011 6(8), e23734. doi: 10.1371/journal.poe.0023734

